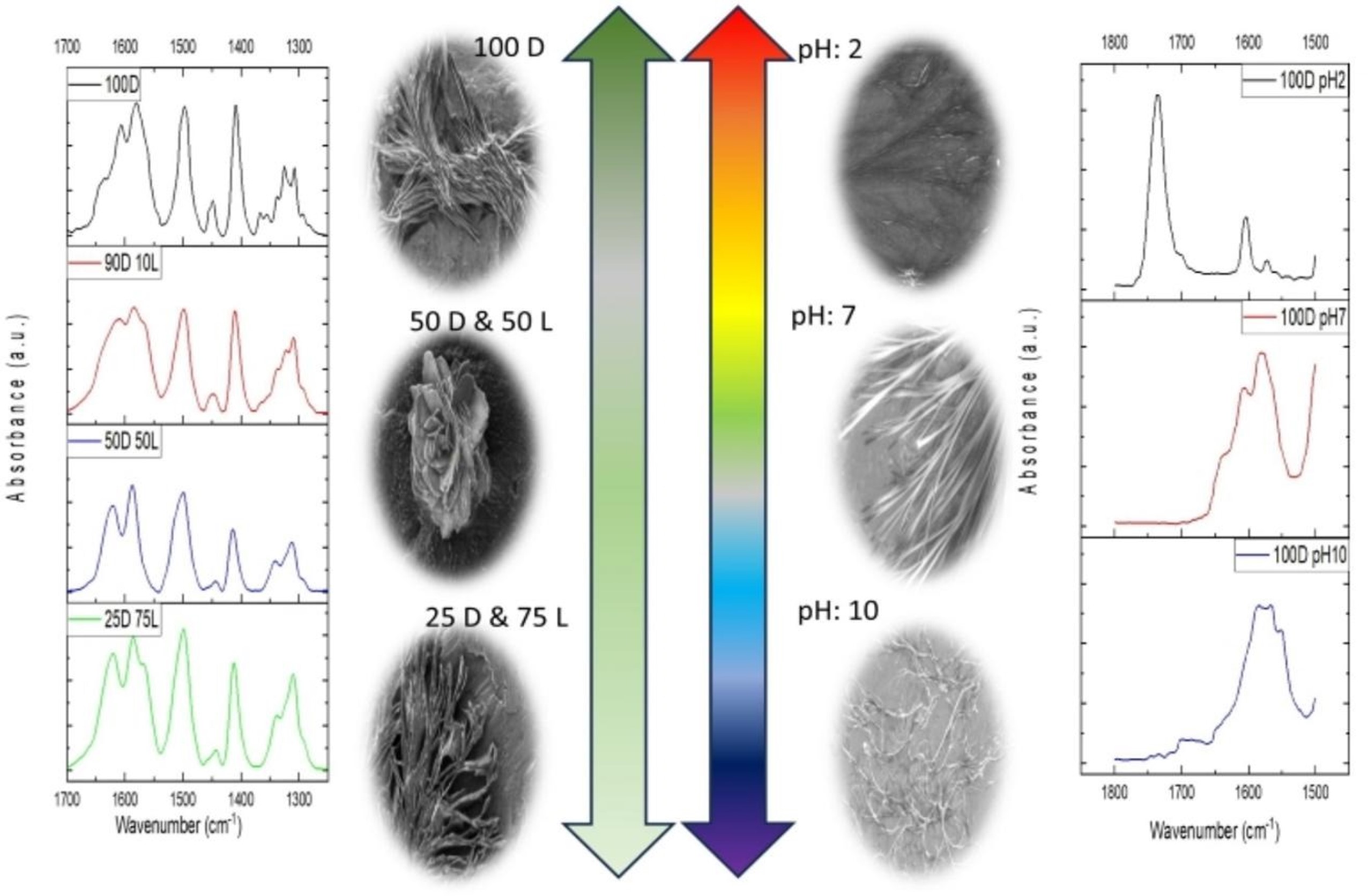
In a recent study published in Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, a team from Istanbul Medeniyet University, Marmara University, and SESAME investigated how L-phenylalanine (L-Phe) fibrils form and become toxic, and how D-phenylalanine (D-Phe) affects this process in different amounts and pH levels.
The study offers new insights into the biochemical mechanisms behind toxic amyloid-like structures—closely linked to neurodegenerative diseases such as phenylketonuria (PKU), Alzheimer’s, and Parkinson’s.
Phenylalanine, a type of amino acid, was found to create needle-shaped fibres at low amounts by using interactions called π-stacking and hydrogen bonding. These structures reduce cell viability in a concentration-dependent manner, mimicking pathological amyloid formation.
Using Synchrotron Fourier-Transform Infrared Microspectroscopy (SR-FTIRµ) at SESAME, researchers could identify specific vibrations related to the formation of harmful fibres.
This advanced technique, which doesn't require labels, allowed for a close look at the molecules in phenylalanine assemblies, showing important chemical signs related to changes in structure. The findings contribute to a deeper understanding of fibril-related toxicity and underscore the value of synchrotron-based techniques for advancing research on amino acid behaviour in disease-relevant contexts.
The full study is available at: https://doi.org/10.1016/j.saa.2025.125891
S. K. Hacıosmanoğlu, M. G. Akkurt, E. Atas, G. Kamel, Ş. Uyaver, M. Kazanci, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 333, 2025