-
Introduction
Hazardous chemicals are any substances that can cause adverse health effects. Not all chemicals are hazardous: non-hazardous chemicals are also termed “non-dangerous goods”. The impact of a hazardous chemical depends on a number of factors, including duration of exposure, frequency of exposure, route of exposure, pre-existing health, and the type of chemical.
Any laboratory that uses chemicals is required to assess and record the hazards associated with those chemicals. The international program for the classification of hazardous substances is the Globally Harmonized System (GHS). GHS has been developed by the United Nations to provide a uniform method of classifying chemicals internationally, and to inform chemical users about chemical hazards they may be exposed to. GHS applies consistent hazard and risk phrases with pictograms for easy recognition on bottles of chemicals. The pictograms below are to be used in combination with other GHS elements that together convey information about the type, severity and management of chemical hazards. GHS is used not just to classify chemicals, but also for labelling and in the detailed description of Safety Data Sheets (SDS).
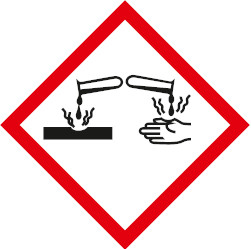

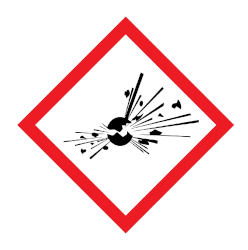 Corrosive – may cause skin burns and permanent eye damage.Irritant / harmful – may cause irritation, skin sensitivity, damage to respiratory tract, acute toxicity.Explosive – May explode if exposed to fire, heat, shock, friction.
Corrosive – may cause skin burns and permanent eye damage.Irritant / harmful – may cause irritation, skin sensitivity, damage to respiratory tract, acute toxicity.Explosive – May explode if exposed to fire, heat, shock, friction.

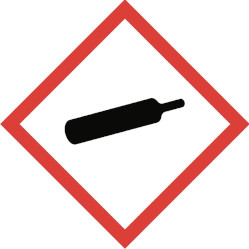 Toxic material – may cause life threatening effects even in small amounts and with short exposure.Flammable if exposed to ignition sources, sparks, heat. Some substances may give off flammable gases.Gases under pressure – gas released may be very cold. Gas container may explode if heated.
Toxic material – may cause life threatening effects even in small amounts and with short exposure.Flammable if exposed to ignition sources, sparks, heat. Some substances may give off flammable gases.Gases under pressure – gas released may be very cold. Gas container may explode if heated.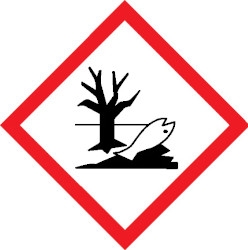

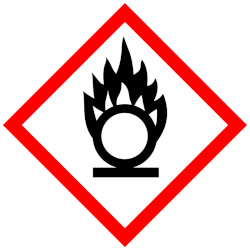 Toxic to aquatic organisms and may cause long-lasting effects in the environmentHealth hazard – may cause serious and prolonged health effects on short- or long-term exposure.Oxidizer – can burn without air, or can intensify fire in combustible materials.
Toxic to aquatic organisms and may cause long-lasting effects in the environmentHealth hazard – may cause serious and prolonged health effects on short- or long-term exposure.Oxidizer – can burn without air, or can intensify fire in combustible materials.Dangerous goods (DGs) are substances that pose a risk to people, property or the environment due to their chemical or physical properties. The hazards they present are immediate and usually quantity related
A Hazardous Substance is any substance (pure or mixed) that has the potential to harm the health of people in the workplace. The term “hazardous” will be included on the bottle label and the SDS will state both hazards and risk phrases. All hazardous substances must be recorded in an inventory or register of hazardous substances, be labeled correctly and have an SDS readily available.
- Safety data sheets
Safety data sheets (SDS), often called material safety data sheets (MSDS) are internationally recognized and provide the information required for the safe handling of hazardous substances. Suppliers are obliged to provide an electronic or hard copy SDS of every chemical sold, so the easiest way to find as SDS is on a supplier’s website. The user is obliged to hold a copy, which is not more than five years old.
There are generally 16 sections in an SDS, although for non-dangerous goods, some sections may not be required. The most significant sections to pay attention to are:
2 - Hazard(s) identification: All hazards regarding the chemical and required label elements.
4 - First-aid measures: Required first aid treatment for exposure to a chemical and the symptoms (immediate or delayed) of exposure.
6 - Accidental release measures: Steps to take in the event of a spill or release involving the chemical. Includes: emergency procedures, protective equipment and proper methods of containment and cleanup.
7 - Handling and storage: Precautions for safe handling and storage, including incompatibilities.
- Chemical storage and transport
Since many of the hazardous materials can react to form a greater hazard, chemical segregation and storage compatibility is essential to safety inside the lab. Dangerous goods (DG) classification is categorized to enable control of transportation, but also applies to storage of materials. Materials are denoted by a DG Class 1-9 with a Packing Group indicating severity of danger (I-III). The Dangerous Goods code is depicted by a colored Dangerous Goods Diamond.
Materials of different DG classes must never be transported in the same container or stored in the same location. Chemical containers must never be transported or stored without secondary containment, in case of spills. In carrying a chemical from one lab to another, a container such as a plastic bucket or tray should be used.
Limiting the volumes of hazardous materials to be used.
The use of alternate or substitute chemicals that are less hazardous.
The size of the reaction vessel in relation to the total volume of reagents and the process(es) to be conducted.
Consideration of decanting small volumes of reagents from larger stock bottles. The risk must include this step, not just the use of the small volume in the reaction.
Special consideration of personal protective equipment (PPE) such as face shield, fit-for-purpose gloves, overalls instead of lab coat.
Methods to release pressure build-up.
Engineering controls: Use of fume hoods or barriers (e.g. blast shield), removal of ignition sources, closure of the roof.CLASS 1
Explosives
e.g. TNT
CLASS 2.1
Flammable gases
e.g. acetylene
CLASS 2.2
Non-flammable, non-toxic gases
e.g. nitrogen
CLASS 2.3
Toxic gases
e.g. chlorine
CLASS 3
Flammable liquids
e.g. petrol
CLASS 4.1
Flammable solids
e.g. sulfur
CLASS 4.2
Spontaneously combustible
e.g. zinc dust
CLASS 4.3
Dangerous when wet
e.g. calcium chloride
CLASS 5.1
Oxidizers
e.g. silver nitrate
CLASS 5.2
Organic peroxides
e.g. methyl ethyl ketone peroxide
CLASS 6
Toxic substances
e.g. sodium cyanide
CLASS 7
Radioactive substances
e.g. uranium
CLASS 8
Corrosives
e.g. hydrochloric acid
CLASS 9
Miscellaneous
e.g. asbestos
- Chemical use
A key aspect of working safely with chemicals is to systematically manage risks that arise from day-to-day operations. There must also be effective consultation and communication within teams and with supervisors about risks and how they are to be managed. In order to do this, a risk assessment should be performed each time a new procedure is planned. Here, the term new procedure includes any variation in the chemical components for an existing procedure, or the modification of the conditions under which an existing process is performed.
The five main steps in a risk management process are:
- Identify hazards and hazardous jobs
- Assign priority for each hazard and hazardous job
- Assess the risk or the potential for the hazard leading to an adverse situation (i.e. what are the consequences?)
- Control the risk(s), preferentially with the view to eliminate them where possible
- Evaluate periodically to check that the risks are being effectively managed
In cases where the Safety Officer determines that a procedure carries too high a risk, the users will be asked to re-write the risk assessment, considering what control mechanisms they can put into place. Control mechanisms to mitigate or eliminate risk will include, but are not limited to:
- Limiting the volumes of hazardous materials to be used.
- The use of alternate or substitute chemicals that are less hazardous.
- The size of the reaction vessel in relation to the total volume of reagents and the process(es) to be conducted.
- Consideration of decanting small volumes of reagents from larger stock bottles. The risk must include this step, not just the use of the small volume in the reaction.
- Special consideration of personal protective equipment (PPE) such as face shield, fit-for-purpose gloves, overalls instead of lab coat.
- Methods to release pressure build-up.
- Engineering controls: Use of fume hoods or barriers (e.g. blast shield), removal of ignition sources, closure of the roof.
-
Use of compressed gas cylinders
Compressed gas cylinders must only be used under the supervision of or with the permission of the beamline scientists. Required PPE is closed shoes, cryogenic gloves and safety glasses.
- General actions in case of contact with chemicals
The SDS will provide specific instructions for the exact chemical in question. Remain calm and do not panic.
In case of a cut or puncture, or chemical contact with the skin, immediately wash the wound under running water for at least 5 minutes.
In case of eye contact, immediately rinse your eyes at the eye wash station for at least 20 minutes.
In case of swallowing, seek medical attention immediately.
Inform the safety officer of all incidents.
Tab

